Thermodynamics
Thermodynamics is about studying temperature, heat and their relation to work, energy, properties of matter and radiation.Absolute temperature
There are different units that we can use to measure a temperature, such as °C, °F. SI unit of temperature is kelvin (K). The kelvin scale is called the absolute temperature scale and the temperature with kelvin unit is called absolute temperature. The absolute temperature starts with 0 K. There is no negative temperature in the kelvin scale, but in Celsius and in Fahrenheit scales you can have both positive and negative temperatures.To convert a temperature from degree celsius to kelvin, just add 273 (precisely it is 273.15) to the celsius temperature.
Ideal gas law
When the pressure is not too high and the temperature is not close to the liquefaction temperature of a gas, all gases obey the following equation, called ideal gas law:$PV=nRT$
where $P$, $V$ and $T$ are the pressure, volume and the temperature of the gas; $n$ is the number of moles in the gas and $R$ is a constant, called the gas constant:$R=8.314 \,J/mol.K$
Note that in the gas law, all the units are in SI unit, so for the temperature, the unit is $K$.
Gases that obey the ideal gas equation are called ideal gases.
Number of moles, $n$ of a gas is the SI unit for the amount of substance, like mass. Number of moles in a given amount of substance is
$n=\dfrac{m}{M}$
where $m$ is the mass of the substance in grams and $M$ is molecular mass (if the substance is a compound made of molecules) or the atomic mass (if the substance is an element made of one type of atom), in grams.
System and surroundings
A thermodynamic system is an object or a group of objects. Surroundings or the environment is everything else in the universe other than the system.Internal energy
Internal energy, U, of a system is the sum of the total energy of all the molecules in the system. For a system of ideal gas,$U=\dfrac{3}{2}nRT$
If heat is added to a system, its temperature increases, so internal energy increases. Internal energy decreases when a system does work, as energy is spent when work is done.
The First Law of Thermodynamics
The first law of thermodynamics is a law of conservation of energy that involves heat transfer. It states that " change in internal energy of a system is equal to the heat added to the system minus work done by the system on its surroundings."i.e., $\Delta U=Q-W$
where $Q$ is the heat added to the system and $W$ is the work done by the system.
Note that, work can be positive or negative, a positive work means work is done by the system, and a negative work means work is done on the system.
Work done by a gas or on a gas
When there is a change in volume of a system, a work has been done. To derive an equation for work, let us consider a cylinder with a piston, containing some gas. Let the gas pressure is P, and is constant. Assume the gas slowly expands against the piston. Due to the expansion of the gas, the piston moves by a distance, d.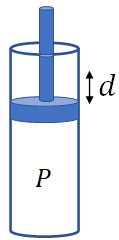
The piston movement is due to the force, $F$ on the piston that results from the gas pressure. If $A$ is the area of the piston, which is also the area of cross section of the cylinder, then the magnitude of the force on the piston is
$F=PA$
The work done by the gas on the piston is
$W=F\,d$
$=P\,A\,d$
But $A\,d$ is nothing but the change in volume, $\Delta V$ of the gas.
Therefore, $W=P\,\Delta V$
This is the work done by the gas when the pressure is constant.
If the gas is compressed instead of expansion, then the volume of the gas decreases. So, ΔV is negative and the work will be negative. When the gas is compressed work is done on the gas by the piston.
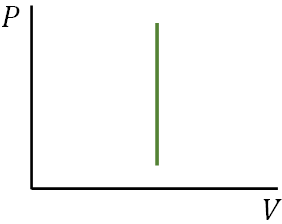
P-V diagram
A diagram showing the plot of pressure, $P$ versus volume, $V$ of a system is called $P$-$V$ diagram. It is useful to analyze thermodynamics processes.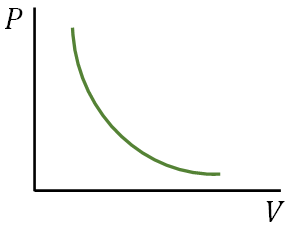
With the P-V diagram, we can find the work done in a thermodynamic process. The work is just the area under the P-V curve.
Thermodynamic processes
A thermodynamic process is the change of state of a system due to the change in the system properties such as pressure, volume and temperature. Following are the common thermodynamic processes.- Isothermal process
- Adiabatic process
- Isobaric process and
- Isovolumetric process
Isothermal process
An isothermal process is a thermodynamic process, where the temperature of the system is constant.When the temperature is constant, ΔT = 0.
So, considering an ideal gas system, the change in internal energy is
$\Delta U=\dfrac{3}{2}nR\, \Delta T = 0$
i.e., There is no change in internal energy of the system.
Therefore, from the first law of thermodynamics,$W = Q$ for an ideal gas system.
Adiabatic process
In an adiabatic process, there is no heat flow into or out of the system,i.e., Q = 0.
So, from first law of thermodynamics,
$\Delta U = -W$
Following is the P-V diagram of an ideal gas during the isothermal and the adiabatic process.
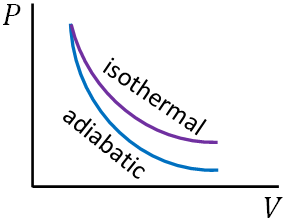
Isobaric process
An isobaric process occurs at constant pressure.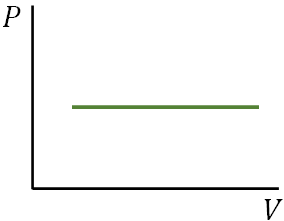
You learned that when the pressure is constant, work done by a gas (system) is
$W=P\,\Delta V$
This is the work done in an isobaric process.
Isovolumetric process
An isovolumetric process occurs at constant volume, (ΔV = 0). So, no work is done by the gas or on the gas as there is no volume change. Therefore,$W=0$
Heat engine
A heat engine produces work using thermal energy. A steam engine is one type of heat engine.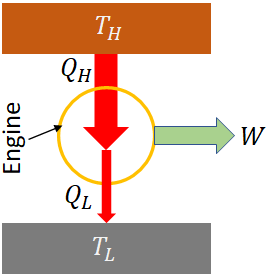
Work done by a heat engine is
$W=Q_H-Q_L$
Efficiency, η of a heat engine is the ratio of the work done by the engine to the heat input,
$\eta=\dfrac{W}{Q_H}=1-\dfrac{Q_L}{Q_H}$
Efficiency of an ideal heat engine is
$\eta=1-\dfrac{T_L}{T_H}$
Temperature’s are absolute temperature (in K).
Even in ideal case, one cannot get a 100% efficiency in a heat engine, as it is not possible to achieve, the temperature, $T_L=0$. So the efficiency is always less than 100 %. Further, real engines have frictional losses, so the best achievable efficiency is 60-80% of the ideal efficiency.
Refrigerators, air conditioners, and heat pumps
These appliances are like heat engines but operating in the reverse. In a heat engine work is done by the engine, but in refrigerators, air conditioners, and heat pumps, work is done on the system to extract heat from the cold reservoir and exhausted to the hot reservoir.Performance of a refrigerator or an air conditioner is measured by the coefficient of performance (COP). It is defined as
COP $=\dfrac{Q_L}{W}$
(or)COP $=\dfrac{Q_L}{Q_H-Q_L}$ ; [since $W=Q_H-Q_L$]
For an ideal case,COP $=\dfrac{T_L}{T_H-T_L}$
Heat pumps are used to heat houses during the winter. Coefficient of performance (COP) of a heat pump is defined asCOP $=\dfrac{Q_H}{W}$
Entropy
Entropy is a thermodynamic quantity that is a measure of the degree of disorder of a system.The entropy, $S$ of a system changes when a heat is added to the system. The change in entropy is
$\Delta S=\dfrac{Q}{T}$
where $Q$ is the heat added to the system and $T$ is the absolute temperature.Second law of thermodynamics
The second law of thermodynamics can be stated in many ways. One statement is"heat cannot flow from cold object to hot object without any external work"
Another statement is"total entropy of an isolated system never decreases, i.e., entropy will either increase or remains constant."
Since entropy is a measure of the disorder of a system, another statement of the second law of thermodynamics is "natural processes tend to move toward a state of higher disorder".
