Heat
Heat is a form of energy. It can transfer from one object to another if they are at different temperatures. Heat transfers spontaneously from an object at a higher temperature to an object at a lower temperature but not in the opposite direction.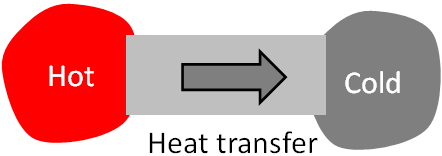
Unit of heat
Since heat is an energy, its SI unit is joules (J). Other common, non-SI unit of heat is calories (cal.). If you want to raise the temperature of an object, you need to add heat to the object. 1 calorie is defined as the amount of heat required to raise the temperature of 1 gram of water by 1 °C, standardized as from 14.5°C to 15.5 °C.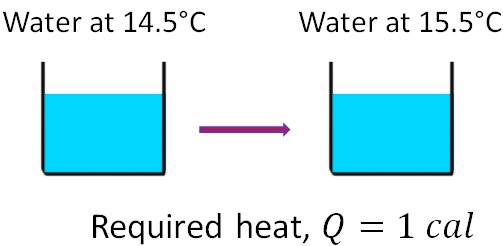
$1 \,cal. = 4.186\, J$
The unit calorie used in food labels is called food calorie:
$1 \,food \:cal. = 1000\, cal.$
Although, 1 cal. is defined as the heat required to raise the temperature of 1 kg of water from 14.5°C to 15.5°C, we can use 1 cal. as the heat required to raise the temperature of 1 kg of water by 1°C for other temperatures too.
Specific heat
To raise the temperature of a substance, a heat must be added to it. But how much heat is required ? It depends on: (1) amount (mass) of the substance, i.e., more substance means more heat requires to raise its temperature, (2) temperature difference $(\Delta T)$ between the object and the heat source and (3) the material of the substance, i.e., some materials require more heat than other to raise the temperature to the same level. If we take into account all these aspects, we arrive at the following equation for the amount of heat $Q$ required to raise the temperature of an object by an amount, $\Delta T$,$Q=mc\Delta T$
where $c$ is called the specific heat of the material of the object. Specific heat is a material property, for water, specific heat is 4186 J/kg/°C and for iron, it is 450 J/kg/°C. Since water has a higher value of specific heat than iron, water requires more heat than iron to increase the temperature by the same amount.Types of systems
There are three types of systems regarding the mass and the energy of the system: open system, closed system and isolated closed system. In an open system, mass and energy can enter or leave the system. In a closed system, there is no transfer of mass in and out of the system, but the energy can transfer. If there is no transfer of mass and energy in or out of the system, then we call that an isolated closed system.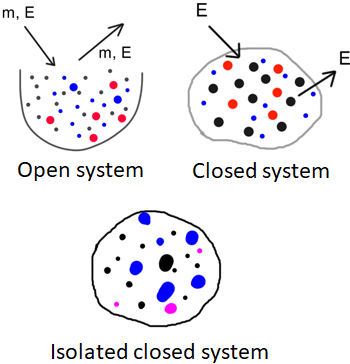
Heat transfer in an isolated closed system
We take the heat, $Q$ as positive, if the heat is added to an object or the object gains heat. And the heat, $Q$ is negative if the heat is removed from the object or the object loses heat.Since in an isolated closed system, there is no transfer of mass and energy in or out of the system, any heat (energy) lost by one object is gained by other objects in the system. That is the amount of heat gain is equal to the amount of heat loss:
$Q_{gain}=-Q_{lost}$
The left hand side, $Q_{gain}$ is a positive number. So, the right hand side also should be positive. Since $Q_{lost}$ is negative, I added a minus sign to make the right hand side positive.
If you add the heat gain and the heat loss, you will get the net heat transfer.
$Q_{net}=Q_{gain}+Q_{lost}$
The right hand side is zero according to the previous equation. Therefore,
$Q_{net}=0$
Thus, the net heat transfer in an isolated closed system is zero.
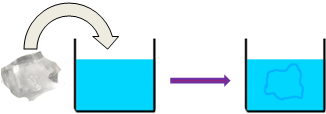
For example, if you add a piece of ice into water, water loses some heat to the ice and the ice gains the heat lost by the water. Here, we assume that the water plus ice system is an isolated system and ignore any heat loss from the system to the surrounding.
Calorimetry and calorimeter
Calorimetry is a technique to quantitatively measure the heat exchange in a system. The instrument used for calorimetry is called a calorimeter. Calorimeter is used to measure the heat exchange and the specific heat capacity of substances. It contains a cup and a stirrer placed in an insulated enclosure to avoid heat exchange with the surroundings. Since there is no heat transfer outside the system, we consider it is an isolated closed system.Latent heat
Latent heat is the heat required to change the phase of a substance. The phase change can be from solid to liquid, liquid to solid or solid to vapor, or in the reverse direction.To understand the latent heat, let us consider a piece of ice at some temperature, say -25°C, and you want to convert the ice into liquid water at say 15°C. That is you need to raise the temperature of the ice from -25°C to 15°C. For that you are adding some heat. If you use a thermometer to measure the temperature of the ice while you heat, you will see the temperature rising until it reaches 0 °C. But once the temperature reaches this temperature, you will not see any change in temperature until all the ice has been melted. So, whatever the heat that you added to the substance at the on set of melting to the complete melting is just used to change the phase of the ice, from solid ice at 0 °C to liquid (water) at 0 °C. Since this heat is not showed as temperature rise, we call this latent heat. Latent means not visible. Latent heat does not increase the temperature of an object, but it only changes the phase of the object.
Latent heat $(L)$ of a substance is defined as the amount of heat required to change the phase of $1 kg$ of the substance. If there is $m$ kg of substance then the required heat for phase change is
$Q=mL$
Since there are three different phases (solid, liquid and gas), there are three different latent heats associated with each phase change: latent heat of fusion, latent heat of vaporization and latent heat of sublimation.
Latent heat of fusion, $L_F$ is defined as the heat required to change the phase of 1 kg of a substance from solid to liquid. So, the heat required to change the phase of m kg of the substance from solid to liquid is
$Q=mL_F$
Latent heat of vaporization, $L_V$ is the heat required to change the phase of 1 kg of a substance from liquid to vapor. So, the heat required to change the phase of m kg of the substance from liquid to vapor is
$Q=mL_V$
Latent heat of sublimation, $L_S$ is the heat required to change the phase of 1 kg of a substance from solid to vapor. So, the heat required to change the phase of m kg of the substance from solid to vapor is
$Q=mL_S$
If the phase of the substance changes in the reverse direction, that is, from liquid to solid, or from vapor to liquid, or from vapor to solid, then the substance releases heat. That is the substance loses heat. The heat released by the substance when its phase changes from liquid to solid is
$Q=-mL_F$
Note that there is a minus in the equation as the substance loses heat.
If the phase change is from vapor to liquid, then
$Q=-mL_V$
And for the phase change is from vapor to solid, then
$Q=-mL_S$
Heat transfer
There are three ways by which heat can transfer from one object to another. They are conduction, convection and radiation.Conduction
When two objects are in contact, heat is transferred between the objects if they are at different temperatures. Such a heat transfer between objects in contact is called conduction. In conduction, heat is transferred by means of molecular collisions. Molecules at the hotter side moves/vibrates at higher rates and collide with neighboring molecules and transfer the heat.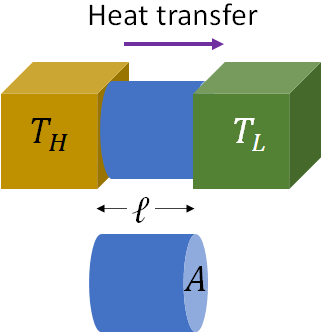
The amount of heat transfer by conduction per unit time is called the heat conduction rate. In the above figure, heat is conducted from an object at a higher temperature, $T_H$ to an object at a lower temperature, $T_L$. The heat is conducted through another object (the blue object) in between them. The heat conduction rate is given by Fourier's equation,
$\dfrac{Q}{t}=kA\:\dfrac{T_H-T_L}{l}$
where $A$ is the area of cross section through which the heat conduction takes place (i.e., the area of cross section of the blue object), $l$ is the distance between the hot and the cold object (length of the blue object), i.e., the thickness of the object in between the two main objects and $k$ is the thermal conductivity of the material through which the heat conduction takes place (the thermal conductivity of the blue object).Thermal conductivity is a material property. For glass, thermal conductivity is 0.84 J/s.m.°C. From the above equation, if you consider, during the winter time, the heat conduction between the inside and outside of a room of a house, the thicker window (i.e., the larger $l$) reduces the heat conduction rate and also the smaller window (i.e., smaller area $A$), reduces the heat conduction through the window.
Convection
Convection is a heat transfer process by bulk movement of molecules from one place to another. For example, at home the heat is carried by the movement of hot air molecules from one place to another from a heater.Radiation
Radiation is the transfer of heat energy from one place to another without any medium in between. For example, the sun transfer heat energy to the earth by radiation. There is no material medium is necessary between the sun and the earth to have sun light here on earth. All objects emit radiation if the temperature is greater than 0 K.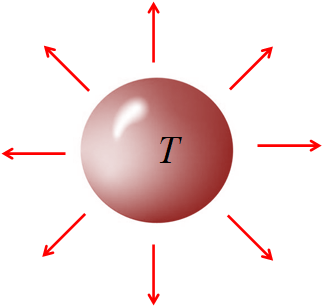
Amount of heat radiated from an object per unit time is called the radiation rate. An object at an absolute temperature $T$ radiates (emits) heat energy at the rate:
$\dfrac{Q}{t}=\epsilon \sigma AT^4$
where $A$ is the surface area of the object, $\epsilon$ is the emissivity of the object, for a perfect emitter $\epsilon =1$. Note that the $T$ is the absolute temperature, i.e., in kelvin.
The above equation is called Stefan-Boltzmann equation.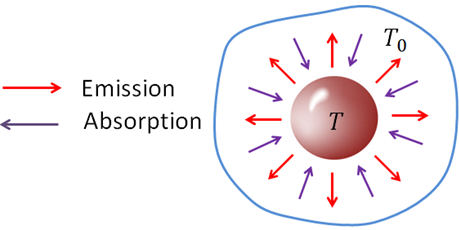
An object that emits radiation also absorbs radiation. Rate of absorption depends on the temperature of the environment. Rate of heat absorbed by an object in an environment of temperature, $T_0$ is
$\dfrac{Q}{t}=\epsilon \sigma AT_0^4$
If you subtract the absorption rate from the radiation (emission) rate, you will get the net radiation rate. Net heat radiation rate of an object at temperature $T$ is therefore,
$\dfrac{Q}{t}=\epsilon \sigma A(T^4-T_0^4)$
