Quantum mechanics of atoms
The Bohr's model of atoms was successful that by which we understand why there is emission and absorption of light by atoms only at certain wavelengths. Further, the wavelengths of the spectra of hydrogen atoms predicted by the Bohr model are in excellent agreement with experiments.Limitations of Bohr's model
In an atomic spectrum, some spectral lines are brighter than others. Further, close inspection of the spectral lines, reveals that there are two or more closed lines within a spectral line. This is called the fine structure. With the Bohr's model we cannot account for the spectral brightness or the fine structure. Although the Bohr's model works good for hydrogen atom in predicting the spectral lines, it is not able to predict the spectra of other complex atoms. Another limitation is, this model cannot explain how atoms are bonded together in molecules.Quantum mechanics
Quantum mechanics is new theory by which we can understand all the features of a line spectrum, the fine structure, spectral brightness and the bonding of atoms in molecules. It provides us a successful model for the simple atom like hydrogen to complex atoms. In short, the quantum mechanics is successful in explaining the microscopic world of atoms, light and its interaction with the subatomic particles.The wave function
In quantum mechanics, a quantity that plays a vital role is known as wave function, $\psi$. It plays different roles in different problems.
- In matter waves, the wave function plays the role of the wave amplitude.
- When considering the electrons as particles, the absolute square of the wave function,$|\psi|^2$ is proportional to the number of electrons at a given point.
- When considering a single electron in an atom, $|\psi|^2$ is the probability of finding the electron at a given point.
Heisenberg uncertainty principle
One of the important principles in quantum mechanics is the Heisenberg uncertainty principle. It states that “we cannot measure both the position and the momentum of an object precisely at the same time.” i.e., if you measure the position of an object more precisely, then your momentum measurement will be less precise and vice versa.If Δx is the uncertainty in the position measurement, and Δpx is the uncertainty in the momentum measurement, then
$\Delta x \, \Delta p_x \ge \hbar$
where $\hbar=\dfrac{h}{2 \pi}$ and $h$ is the Planck's constant.Like position and momentum, there is an uncertainty principle for energy and time, that is
$\Delta E \, \Delta t \ge \hbar$
where ΔE and Δt are the uncertainties in the energy and the time respectively.Note that maximum possible accuracy of a measurements is obtained with the equal sign in the uncertainty principle equations.
Quantum mechanical model of atoms
In Bohr's model of atom, the electrons of an atom revolves around the nucleus in well defined orbits. But according to quantum mechanics, electrons do not orbit in well-defined circular orbits, but they spread out in space as a cloud. That means, here the electrons behave as waves. So, the electron has no definite location. The orbit of an electron is where the probability of finding the electron is the highest.Quantum mechanics of the hydrogen atom
Quantum mechanics predicts the same energy levels for the hydrogen atom as predicted by Bohr’s theory. Energy levels of a hydrogen atom is,$E_n=\dfrac{-13.6\,eV}{n^2}$; $n=1,2,3,...$
where $n=1,2,3,...$ is the principal quantum number.The electron of a hydrogen atom, can be in any one of the levels around the nucleus.
State of electrons and quantum numbers
According to quantum mechanics, four different quantum numbers are needed to specify each state in an atom. An electron in the atom can be in any one of the states.The four quantum numbers are
- Principal quantum number, $n$
- Orbital quantum number, $\ell$
- Magnetic quantum number, $m_{\ell}$
- Spin quantum number, $m_s$
The quantum numbers, their values and what they represent are as follows:
The principal quantum number, $n$ can have any value from 1 to infinity.
Total energy of a state depends on the principal quantum number.
The orbital quantum number, $\ell$ can have integer values from $0$ to $n-1$.
For example, for $n=5$, $\ell=0,1,2,3$, or $4$.
$L=\sqrt{\ell (\ell+1)}\:\hbar$
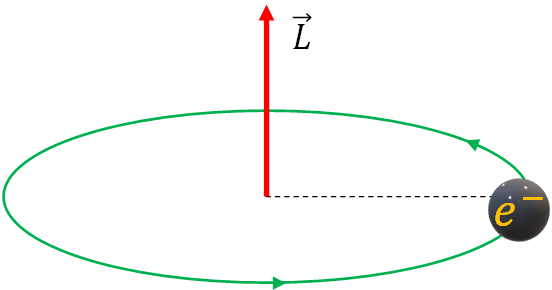
The magnetic quantum number, $m_{\ell}$ can have integer values from $-\ell$ to $+\ell$.
For $\ell=2$, $m_{\ell}$ can have $−2, −2, 0, 1$ or $2$.
The magnetic quantum number represents the direction of the angular momentum of the electron. Each value of $m_\ell$, corresponds to a direction of the angular momentum.
When a gas is placed in a magnetic field, its spectral lines are split into several very closely spaced lines. This splitting of spectral lines is known as Zeeman effect. Magnetic quantum numbers, $m_\ell$ account for these lines.
Spin quantum number, can have only two values
i.e., $m_s=+\dfrac{1}{2}$ and $m_s=-\dfrac{1}{2}$
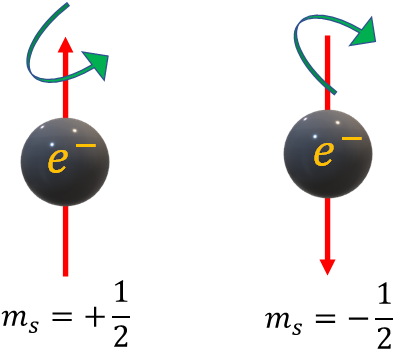
Spin quantum numbers are associated with the spin angular momentum of electrons. Since spin angular momentum can have two directions (up or down), there are two spin quantum numbers.
These quantum numbers account for the fine structure of the spectral lines.
Ground and excited states of an atom
Lowest energy state or level of an atom is called the ground state of the atom. At the ground state of an atom all the electrons of the atom has the lowest energy. Higher energy states (states other than the ground state) are called excited states. Ground state is the normal state of an atom. When an atom absorbs a photon, an electron of the atom makes a transition to a higher energy state (excited state). An electron cannot stay in the excited state, so it make a transition back to the ground state, during this process a photon is emitted. Note that when electrons make transition, they behave like particles.Selection rules and allowed transitions
When a photon is emitted or absorbed by an atom, an electron in the atom makes a transition from one state to another. But not all transitions are allowed. Only transition between states with ℓ values such that$\Delta \ell =\pm 1$
is allowed, called allowed transition, and all other transitions are called forbidden transitions.Pauli’s exclusion principle
Each electron in an atom occupies a particular state characterized by the four quantum numbers. But there is one restriction based on a principle called Pauli’s exclusion principle. It states that, “No two electrons in an atom can occupy the same quantum state” In other words, no two electrons in an atom can have all the quantum numbers same, at least one of the quantum numbers must be different.Electron configuration of elements
Arrangement of electrons in an atom of an element is called electronic configuration of the element.To find the electronic configuration, we use the quantum numbers and the Pauli’s exclusion principle.Each principal quatum number, $n$ represents a shell.
$n=1$ is called $K$ shell; $n=2$ the $L$ shell; $n=3$ the $M$ shell and so on.
The electrons with the same value of $n$, are in the same shell.The $\ell$ value represents a subshell.
$\ell=0$ is the $s$ subshell
$\ell=1$ is the $p$ subshell
$\ell=2$ is the $d$ subshell
$\ell=3$ is the $f$ subshell
then, $g$, $h$, $i$ and so on.
Electrons with same values of $n$ and $\ell$ are in the same subshell.
For a given subshell of $\ell$, there can be a maximum of $2(2\ell+1)$ electrons.
Example: The electronic configuration of sodium:
Atomic number of sodium is $Z=11$. i.e., sodium has 11 electrons.Its electronic configuration is
$1s^2\: 2s^2\: 2p^6\: 3s^1$
We first write the shell number followed by the subshell, and put the number of electrons in the subshell as a superscript. The number of electrons in a subshell cannot exceed $2(2\ell+1)$.
